CUET (UG) Section II Science Group Domain subject Chemistry Sample Paper for online practice. Chemistry Mock Test as per latest syllabus and exam pattern for the admission in 2024 – 2025 academic session.
CUET (UG) Sample Paper : Chemistry
Instruction : Attempt any 40 questions, out of 50
Duration : 45 Minutes
Q.1: The lattice points of a crystal of hydrogen iodide are occupied by
(a) HI molecules
(b) H atoms and I atoms
(c) H+ cations and I− anions
(d) H2 molecules and I2 molecules
Show Answer
Q.2: The number of octahedral void(s) per atom present at a cubic close packed structure is
(a) 1
(b) 3
(c) 2
(d) 4
Show Answer
Q.3: Which one is not a ferroelectric compound?
(a) KH2PO4
(b) K4[Fe(CN)6]
(c) Rochelle salt
(d) BaTiO3
Show Answer
Q.4: What is the percentage of solute in the resultant solution, if it is obtained by mixing 300g of 30% and 200g of 20% solution by weight?
(a) 50%
(b) 26%
(c) 62%
(d) 32%
Show Answer
Q.5: The examples of minimum boiling azeotropes are
(a) aniline + acetone
(b) acetic acid + pyridine
(c) HCl + water
(d) cyclohexane + ethanol
Show Answer
Q.6: At 300 K two pure liquids A and B have 150 mm Hg and 100 mm Hg vapour pressures, respectively. In an equimolar liquid mixture of A and B, the mole fraction of B in the vapour mixture at this temperature is
(a) 0.6
(b) 0.5
(c) 0.8
(d) 0.4
Show Answer
Q.7: A 4.0 M aqueous solution of NaCl is prepared and 500 mL of this solution is electrolysed. This leads to evolution of chlorine gas at one of the electrodes. The total charge required for the complete electrolysis will be
(a) 96500 C
(b) 24125 C
(c) 48250 C
(d) 193000 C
Show Answer
Q.8: Galvanisation is
(a) zinc plating on aluminium sheet
(b) zinc plating on iron sheet
(c) iron plating on zinc sheet
(d) aluminium plating on zinc sheet
Show Answer
Q.9: Zn (s) + Cu2+ (aq) ![]() Zn2+ (aq) + Cu(s)
Zn2+ (aq) + Cu(s)
The above redox reaction is used in
(a) Galvanic cell
(b) Daniell cell
(c) Voltaic cell
(d) All of these
Show Answer
Q.10: Rate law cannot be determined from balanced chemical equation, if ……… .
(a) reverse reaction is involved
(b) it is an elementary reaction
(c) it is a sequence of elementary reactions
(d) Both (a) and (c)
Show Answer
Q.11: A first order reaction has a rate constant of 2303×10-3 S-1 . The time required for 40 g of this reactant to reduce to 10 g will be [Given that log10 2=0.3010]
(a) 230.3 s
(b) 301 s
(c) 2000 s
(d) 602 s
Show Answer
Q12: 99% completion of a first order reaction takes place in 32 min. The time taken in 99.9% completion of the reaction will be
(a) 48 min
(b) 52 min
(c) 56 min
(d) 44 min
Show Answer
Q.13: In the cleansing action of soaps represented by the
given figures,
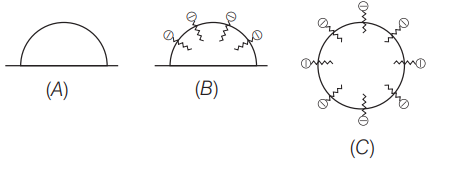
Figure ‘C’ show the structure of
(a) aggregated colloids
(b) macromolecular colloids
(c) Both (a) and (b)
(d) multimolecular colloids
Show Answer
Q.14: Smoke is precipitated by the
(a) Cottrell precipitator
(b) colloidal precipitator
(c) natural precipitator
(d) Planck’s precipitator
Show Answer
Q.15: Butter and cream are the examples of
(a) w/o type emulsion
(b) o/w type emulsion
(c) Both (a) and (b)
(d) None of these
Show Answer
Q.16: Which one of the following is a mineral of iron?
(a) Malachite
(b) Cassiterite
(c) Pyrolusite
(d) Magnetite
Show Answer
Q.17: Sulphide ores are common for which of the following metals.
(a) Ag, Cu and Pb
(b) Ag, Cu and Sn
(c) Ag, Mg and Pb
(d) Al, Cu and Pb
Show Answer
Q.18: In electrolytic refining method,
(a) the impure metal is made to act as anode
(b) a strip of the pure metal is used as cathode
(c) anode and cathode are kept in a suitable electrolytic bath containing soluble salt of the same metal
(d) All the above are true
Show Answer
Q.19: Which of the following hydrides has the lowest boiling point?
(a) PH3
(b) AsH3
(c) SbH3
(d) NH3
Show Answer
Q.20: Which of the following oxides is amphoteric in nature?
(a) Cl2O7
(b) Na2O
(c) N2O
(d) Al2O3
Show Answer
Q.21: Which of the following are the applications of dinitrogen gas?
(a) Preservation of biological materials and food items
(b) Production of inert atmosphere in copper and steel industry
(c) In the preparation of explosives
(d) Etching of metals
Show Answer
Q.22: The third ionisation enthalpy is minimum form
(a) Mn
(b) Ni
(c) Co
(d) Fe
Show Answer
Q.23: The actinoids resemble the lanthanoids in having more compounds in
(a) +3 state
(b) +4 state
(c) +5 state
(d) +2 state
Show Answer
Q.24: Dichromates are generally prepared by the fusion of chromite ore with
(a) sodium carbonate
(b) potassium carbonate
(c) Both (a) and (b)
(d) Neither (a) nor (b)
Show Answer
Q.25: How many ions obtain after dissociation of this complex [Co(NH3)6]Cl3?
(a) 3
(b) 2
(c) 5
(d) 4
Show Answer
Q.26: An example of a sigma bonded organometallic compound is
(a) ruthenocene
(b) Grignard’s reagent
(c) ferrocene
(d) cobaltocene
Show Answer
Q.27: For lead-poisoning, the antidote used is
(a) white of an egg
(b) cis-platin
(c) nickel
(d) EDTA
Show Answer
Q.28: Which of the following is an example of vic-dihalide?
(a) Dichloromethane
(b) 1,2-dichloroethane
(c) Ethylidine chloride
(d) Allyl chloride
Show Answer
Q.29: What is the nature of KCN and AgCN compounds?
(a) Ionic and covalent
(b) Ionic and ionic
(c) Covalent and ionic
(d) Covalent and covalent
Show Answer
Q.30: Which of the following is used to prepare alkyl chloride in presence of alcohol?
(a) H2SO4
(b) HCl solution (dilute)
(c) dry HCl gas
(d) None of these
Show Answer
Q.31: Phenols show the cleavage of C— O bond with
(a) Na
(b) K
(c) Zn
(d) Ca
Show Answer
Q.32: The order of reactivity of hydrogen halides with ether is as follows :
(a) HBr > HI > HCl
(b) HCl > HBr > HI
(c) HI > HBr > HCl
(d) HCl > HI > HBr
Show Answer
Q.33: The action of zymase is inhibited during fermentation if the percentage of alcohol formed exceeds
(a) 5%
(b) 7%
(c) 10%
(d) 14%
Show Answer
Q.34: Carbonyl compounds are the constituents of
(a) fabrics
(b) flavouring
(c) plastics and drugs
(d) All of these
Show Answer
Q.35: Which of the following compounds produces an orange-red precipitate with 2, 4-DNP reagent?
(a) Acetamide
(b) Dimethyl ether
(c) Butanone
(d) Propylbutanoate
Show Answer
Q.36: Which of the following acid is used in rubber, textile, dyeing, leather and electroplating industries?
(a) Hexanedioic acid
(b) Ethanoic acid
(c) Methanoic acid
(d) Sodium benzoate
Show Answer
Q.37: Name the product(s) formed during the reaction of primary aliphatic amines with nitrous acid at room temperature?
(a) R NO2
(b) ROH
(c) Both (a) and (b)
(d) None of these
Show Answer
Q.38: Coupling reaction is an example of
(a) nucleophilic addition reaction.
(b) nucleophilic substitution reaction.
(c) electrophilic substitution reaction.
(d) electrophilic addition reaction.
Show Answer
Q.39: The chemical formula of Hinsberg’s reagent is
(a) HNO2
(b) NaOH+CaO
(c) C6H5SO2Cl
(d) CH3CONH2
Show Answer
Q.40: Invert sugar is a mixture of
(a) D-glucose + D-fructose
(b) L-glucose + D-fructose
(c) L-glucose + D-glucose
(d) L-glucose + L-glucose
Show Answer
Q.41: Which of the following is not a hormone?
(a) Insulin
(b) Endorphins
(c) Norepinephrine
(d) Thymine
Show Answer
Q.42: Water soluble vitamin is
(a) vitamin C
(b) vitamin D
(c) vitamin E
(d) vitamin K
Show Answer
Q.43: Buna-S is a
(a) natural polymer
(b) synthetic polymer
(c) semi-synthetic polymer
(d) None of these
Show Answer
Q.44: Biodegradable polymer which can be produced from glycine and amino caproic acid is
(a) nylon-2-nylon-6
(b) PHBV
(c) buna-N
(d) nylon-6, 6
Show Answer
Q.45: The type of polythene which is chemically inert and more tough and hard is
(a) HDP
(b) LDP
(c) Both (a) and
(b) (d) None of these
Show Answer
Q.46: The receptor proteins are embedded in the
(a) DNA
(b) cell membrane
(c) cytoplasm
(d) RNA
Show Answer
Q.47: Food preservatives prevent spoilage of food due to microbial growth. The most commonly used preservative is
(a) C6H5COONa
(b) table salt
(c) vegetable oils
(d) All of the above
Show Answer
Q.48: Noradrenaline is a/an
(a) antidepressant
(b) antihistamine
(c) neurotransmitter
(d) antacid
Show Answer
Q.49: Which lanthanoid does not occur naturally?
(a) Eu
(b) Pm
(c) Gd
(d) Lu
Show Answer
Q.50: Which primitive unit cell has unequal edge lengths ![]() and all axial angles different from 90°?
and all axial angles different from 90°?
(a) Hexagonal
(b) Monoclinic
(c) Tetragonal
(d) Triclinic
Show Answer
Answer Key : CUET (UG) Chemistry Sample Paper
| 1. (a) | 2. (a) | 3. (b) | 4. (b) | 5. (d) | 6. (d) | 7. (d) | 8. (b) | 9. (d) | 10. (b) |
| 11. (d) | 12. (a) | 13. (a) | 14. (a) | 15. (a) | 16. (d) | 17. (a) | 18. (d) | 19. (a) | 20. (d) |
| 21. (a) | 22. (d) | 23. (a) | 24. (c) | 25. (d) | 26. (b) | 27. (d) | 28. (b) | 29. (a) | 30. (c) |
| 31. (c) | 32. (c) | 33. (d) | 34. (d) | 35. (c) | 36. (c) | 37. (b) | 38. (c) | 39. (c) | 40. (a) |
| 41. (d) | 42. (a) | 43. (b) | 44. (a) | 45. (a) | 46. (b) | 47. (d) | 48. (c) | 49. (b) | 50. (d) |
Thanks for the visit and attempt CUET (UG) Chemistry Sample Paper.
You may find more CUET (UG) Chemistry Sample Paper : CUET Sample Paper Archives